

In recent years, more and more people advocate the "3Rs" principle of experimental animals: Reduction, Replacement, and Refinement. For ethical, scientific, and legal considerations, choosing an appropriate humane endpoint that minimizes adverse effects on animals is an important part of "Refinement" in the 3Rs. While the ‘experimental endpoint’ of a study occurs when the scientific objectives have been achieved, the ‘humane endpoint’ is the moment at which pain or distress in an experimental animal is prevented, terminated, or relieved.
For high-quality oncology research, reasonable humane endpoints can reduce non-specific systemic effects, thereby increasing the accuracy of the results and providing the highest level of animal welfare. For experimental animals in oncology program research, health and welfare are unquestionably placed first.
So how do scientists determine humane endpoints in animal research for oncology? Let’s learn together!
First of all, it is stated that the humane endpoints presented in this passage are based on widely used animal experiments. The characteristics of the progress of the experiment itself should be considered to control for humane endpoints if we specify each study. For example, carcinogenicity studies can be terminated once the tumor has progressed significantly; conversely, some tumors do not undergo malignant transformation until later in their development, and humane endpoints need to be delayed, such as carcinogen-induced skin papilloma.
① Pre-experiments, to determine the complete extent of tumor development and progression, will help to identify reliable and precise humane endpoints.
Humane endpoints for a particular model must also take into account the known pathogenesis of that particular tumor model and should be periodically reviewed empirically. Imaging techniques can help provide more defined humane endpoints for the development of certain tumor models. Every effort should be made to identify the various influencing factors, combined with the impact on animal welfare, to make early scientific decisions. Deliberately ending with tumor-induced death is unacceptable behavior.
② The choice of location for solid tumor engraftment will influence the maximum acceptable tumor burden and appropriate humane endpoints.
(a.) Implantation of tumors into areas such as footpads, tail, eyes, or bones may make the animal more sore or painful, requiring special handling and an earlier humane endpoint. Likewise, tumors that metastasize to sensitive sites need to be very careful.
(b.) Weight loss has been reported as a humane endpoint. If a brain tumor is necessary, such as to improve understanding of brain tumor biology and develop treatments, MRI or bioluminescence imaging (BLI) techniques may be very useful.
(c.) Intramuscular tumors are painful and orthotopic transplantation should only be performed when in situ studies (e.g., sarcomas) are justified.
③ For transgenic animals, special care is required to ensure the timely detection of unexpected sites of tumor development.
As with all internal tumor sites, this includes clinical examination, weight measurement, abdominal palpation, and status changes. Discussions must be held between researchers, veterinarians, and animal husbandry staff to determine humane endpoints, professional feeding management, and interventions before the experiment begins. Obtaining detailed phenotypic information by testing and evaluating experimental analytical data (e.g., pharmacodynamic assays, functional imaging) can help to identify plausible endpoints.

In clinical practice, tumor burden refers to the degree of harm of a tumor to the body. In general, the assessment includes tumor size, tumor activity, tumor metastasis, and the degree of harm caused by the location of the tumor in the body. Assessment of tumor size and burden is an important basis for determining humane endpoints.
① Tumor burden should always be limited to the minimum required for valid scientific results
Efficacy studies should be terminated once they show a durable, statistically significant treatment effect. The design of therapeutic studies should control tumor volume and avoid tumors that are too large. Any tumor should be limited in size when used solely for routine transplantation or as a source of tumor tissue. In all cases, the general health and condition of the animal remain the most important determinant. Adverse effects on the animal will depend on the biology, transplant site, how the tumor grew, and any additional manipulations or treatments.
For animals carrying a single tumor, the average tumor diameter should not exceed 1.2 cm in mice and 2.5 cm in rats under normal conditions; if used for therapeutic preclinical studies, they should not exceed 1.5 and 2.8 cm, respectively. For example, where there are two tumors on the contralateral side of each animal, the tumor size should be reduced accordingly and should not exceed the maximum burden of a single tumor. Similar limitations should be observed in transgenic animals that may develop a variety of tumors or in experiments conducted on the skin of animals exposed to UV radiation or chemical carcinogens. If there are special requirements to exceed the recommended tumor size limit, it should undergo rigorous scientific evaluation.
② Using vernier calipers to measure the size of superficial tumors is a simple and easy method
Ensuring that the technician is skilled and remains throughout the study period can minimize measurement bias. Response to treatment can be measured by changes in tumor growth rate, delay in re-growth, cell survival (measured by clonal assays), or appropriate surrogate markers. Tumors were removed and weighed at the end of the study to provide an additional objective endpoint, which could reduce errors due to changes in tumor shape and assessment of volume or mass.
③ Pre-experiments and observation of surrogate markers are effective aids
It is challenging to determine the tumor burden of internal carcinoma in situ, systemic lymphoreticular tumor, or metastatic disease. Pre-experiments using a small number of animals are important for determining tumor development kinetics and patterns, predicting clinical symptoms, and identifying humane endpoints. Biomarkers or circulating cancer cells can be used as surrogates for assessing lymphoma and leukemia burden, and monitoring these surrogates with real-time imaging is an effective adjunct. We should use appropriate biochemical and pathological indicators, engineered reporting systems, or imaging techniques to identify disease onset. Ongoing humane endpoint analysis must also be assessed based on the general condition of the animal, as well as palpable tumors and specific signs such as hindlimb weakness or paralysis.
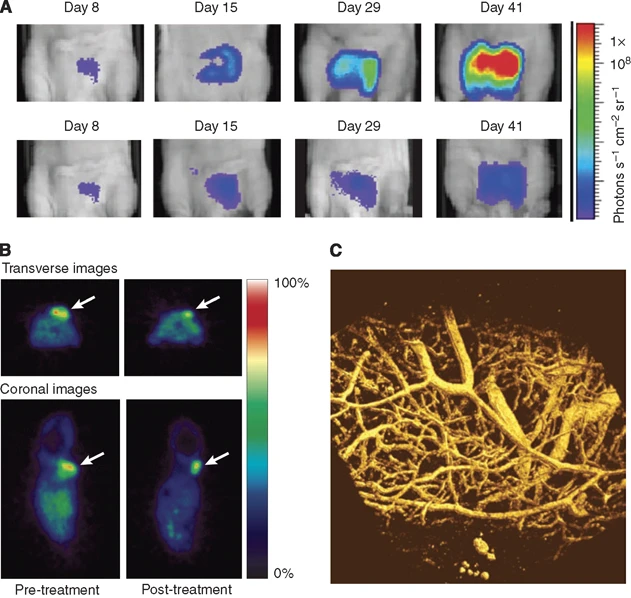
Figure 2. Examples of in vivo imaging in preclinical cancer research [1]
In general, the clinical signs shown below are the main indicators of rare but serious symptoms of potential adverse reactions. Such serious symptoms may be rare in well-designed experimental studies, which should be avoided. As soon as such severe symptoms are detected, humane endpoints should be implemented immediately and other animals in the study should be observed intensively.
Animal clinical signs requiring immediate intervention:
❖ Not eating or drinking for 24-48 hours, leading to weight loss or dehydration.
❖ Sustained or rapid weight loss of 20% or 15% over 72 hours compared with pre-experimental adult mice or with control animals of the same age. However, for some tumors, weight is not a good indicator, and muscle wasting or wasting can be used as an evaluation indicator. The Body Condition Score is a very useful indicator of muscle loss.
❖ Persistent hypothermia.
❖ Bloody or mucopus discharge from any natural orifice.
❖ Difficulty breathing, especially with a runny nose and/or cyanosis.
❖ Enlarged lymph nodes or spleen.
❖ Paralysis or weakness of the hind limbs.
❖ Anemia, symptoms such as pale feet or hematological markers.
❖ Significant abdominal distension or ascites burden greater than 10% of body weight, compared to control animals of the same age. But this indicator is difficult to measure accurately and can be replaced by measuring waist circumference, which usually allows up to a 20% increase, similar to the body size of a pregnant one.
❖ Urinary incontinence or diarrhea for more than 48 hours.
❖ Tumors that affect movement or cause abnormal vocalization, animal behavior, or function.
In addition, for solid tumors, the presence of ulceration, overlying tissue swelling, and cachexia (severe weight loss) should be included in the evaluation of humane endpoints. An ulcer is a typical lesion of superficial tissue necrosis, which may be dry, purulent, or oozing. Necrosis leading to skin rupture or exudation lasting more than 48 hours can be considered a humane endpoint. Some tumors, such as those that grow in sensitive sites or develop extensive necrosis, can be distressing to animals, despite the lack of objective criteria in the animal. Further research is needed to better assess pain and assist in developing the most appropriate humane endpoints for such cases.
Humane endpoints should be specified and actions should be taken to safeguard animal welfare before research begins. Pre-experiments to determine the degree of tumor development in particular models, especially for transgenic lines, is essential to obtain the phenotypic data necessary to develop a fully-informed humane endpoint strategy for your oncology research. Regardless of whether or not the experimental objectives have been achieved, animal welfare must be upheld through humane endpoints: when the level of animal suffering required by scientific objectives has been exceeded, scientific objectives have been achieved (or cannot be achieved), or when the quality of experimental results cannot be maintained.
Reference:
[1]P Workman, E O Aboagye, F Balkwill, et al. Guidelines for the welfare and use of animals in cancer research[J]. British Journal of Cancer (2010) 102, 1555 – 1577.




